|
For the last few years,
scientists in the Department
of Pathology have been
engaged in a project that
revolves around a unique
mouse serendipitously
discovered at Wake Forest
University School of
Medicine in 1999 (the SR/CR
Mouse). The original mouse
of this type was part of an
experiment in which mouse
cancer cells were being
transplanted into the
abdominal cavity of other
mice to produce cancer. This
particular mouse, a member
of a highly inbred strain of
mice (BALB/c, all of whom
 are
essentially identical twins
genetically), did not
develop the expected tumor,
even though it was injected
with a large number of tumor
cells. When it was injected
many times more with these
cancer cells, it still
failed to develop a tumor
(Figure 1). Since the cancer
cells used for the
injections were from an
extremely aggressive type of
cancer (Sarcoma180, a tumor
derived from connective
tissues of a mouse), this
result was highly unusual.
To determine if this
resistance was genetic, this
mouse was bred to other
normal mice, and it was
shown that this resistance
to cancer was inherited. The
pattern of inheritance
showed that it probably was
caused by a single mutation
in a single gene and was
“dominant” (it only required
one copy to work). are
essentially identical twins
genetically), did not
develop the expected tumor,
even though it was injected
with a large number of tumor
cells. When it was injected
many times more with these
cancer cells, it still
failed to develop a tumor
(Figure 1). Since the cancer
cells used for the
injections were from an
extremely aggressive type of
cancer (Sarcoma180, a tumor
derived from connective
tissues of a mouse), this
result was highly unusual.
To determine if this
resistance was genetic, this
mouse was bred to other
normal mice, and it was
shown that this resistance
to cancer was inherited. The
pattern of inheritance
showed that it probably was
caused by a single mutation
in a single gene and was
“dominant” (it only required
one copy to work).
The SR/CR Mouse Colony and
Its Genetics
After the initial
experiments, breeding
studies allowed this gene
mutation to be passed on to
a large number of offspring
from the original mouse
through multiple
generations. In addition to
the original BALB/c mouse
strain (an inbred laboratory
white mouse), the cancer
resistance could also be
bred into other inbred
strains of mice with
different genetic
backgrounds (C57BL/6,
CAST/Ei). Since such mouse
types have been extensively
studied for many years,
scientists have techniques
that can distinguish the
portions of their DNA with
great accuracy and determine
from which mouse strain the
DNA originated. Using this
“genomics” strategy, the
cross-breeding of the
original BALB/c SR/CR mice
to normal C57BL/6 mice
allowed the determination of
which of the mouse
chromosomes carried this
cancer resistance gene.
Further studies will allow
the precise mutation in the
gene involved to be
identified, although this is
a highly complex process.
Does the Resistance Gene in
SR/CR Mice Work Against
Other Types of Tumor?
An important initial
question in studying these
mice was whether the
resistance to cancer only
worked against this unusual
tumor type (S180 sarcoma),
or would work against other
types of cancer. Using
several different mouse
cancer types, such as
leukemia, lymphoma, liver
cancer, and lung cancer, it
was shown that the SR/CR
mouse was resistant to all
of them. Further, other
experiments were done to
show that, in addition to
tumors in the abdominal
cavity, tumors that grow in
other sites, such as under
the skin, were also rejected
by this mouse.
What Happens to the Tumor
Cells Injected into these
Resistant Mice?
Because the initial
experiments used tumor cells
that were transplanted by
injection into the abdominal
cavity of mice, the injected
cells could be recovered at
a later time and examined.
It was also possible to see
if other cell types from the
resistant mouse were
interacting with the tumor
cells directly. When this
was done, it was found that
injected cancer cells in
these mice were killed
within the first day after
they were injected. In
addition, other cells from
the resistant mouse, mostly
white blood cell types, were
found attached to these
cancer cells prior to their
death forming “rosettes”
around the tumor cell.
(Figure 2).
 The
white blood cell types found
in these rosettes included
polymorphonuclear leukocytes
(a common white blood cell
involved in killing bacteria
in infections, also called
“polys”, “neutrophils” or
“PMN’s”), monocytes (a
common white blood cell type
that can also crawl into
tissues where it is called a
“macrophage”) and a special
type of immune cell called a
“natural killer cell” (NK
cell). All of these cells
are part of what is referred
to as the “innate immune
system”, cells that are
active against many foreign
organisms, such as bacteria,
viruses and fungi, without
prior immunization.
Suprisingly, very few of
another type of white blood
cell -- lymphocytes (T cells
or B cells) were found. Such
cells are normally part of
the rejection of foreign
cells by the immune system,
and are frequently involved
in more familiar cell
rejection events, such as
those seen in the rejection
of poorly-matched tissues
and organs (kidney, bone
marrow or skin transplants).
Clearly, something other
than normal tissue
transplant rejection was
happening in these
cancer-resistant mice. The
white blood cell types found
in these rosettes included
polymorphonuclear leukocytes
(a common white blood cell
involved in killing bacteria
in infections, also called
“polys”, “neutrophils” or
“PMN’s”), monocytes (a
common white blood cell type
that can also crawl into
tissues where it is called a
“macrophage”) and a special
type of immune cell called a
“natural killer cell” (NK
cell). All of these cells
are part of what is referred
to as the “innate immune
system”, cells that are
active against many foreign
organisms, such as bacteria,
viruses and fungi, without
prior immunization.
Suprisingly, very few of
another type of white blood
cell -- lymphocytes (T cells
or B cells) were found. Such
cells are normally part of
the rejection of foreign
cells by the immune system,
and are frequently involved
in more familiar cell
rejection events, such as
those seen in the rejection
of poorly-matched tissues
and organs (kidney, bone
marrow or skin transplants).
Clearly, something other
than normal tissue
transplant rejection was
happening in these
cancer-resistant mice.
More Evidence that the
Cancer Resistance is Not Due
to Normal Tissue Rejection
Rejection of transplanted
organs between two
individuals that are not
precisely “matched” (that
is, are not identical twins)
involves a special group of
white blood cells called
“T-lymphocytes.” Such T
cells (T stands for
“thymus”, the organ involved
in their maturation) have
been extensively studied
over the past 30 years, and
can detect small differences
between cells from different
types of mice, and could
have been responsible for
the rejection of the cancer
cells that originally came
from another mouse. In other
words, it was possible that
cancer cells were rejected,
not because they were
cancer, but because they
were foreign. To rule this
out, a cross-breeding
experiment was done, in
which one mouse with the
SR/CR cancer resistance
trait was bred with a mouse
that was genetically
deficient in T cell function
(a so-called “nude” mouse,
since these immune deficient
mice fail to grow hair
normally). Several
generations later, the
offspring from that mating
including some mice that had
the SR/CR cancer resistance
trait, yet were still
defective in normal organ
rejection. They were “nude”
and thymus-deficient, and
yet were still
cancer-resistant. This
showed that the cancer
resistance mechanism
operates even in a mouse
that cannot reject
mismatched organ
transplants. Thus the
resistance mechanism doesn't
use T-cells. This is not
totally a surprise, however,
since these “nude” SR/CR
mice,still have other cells
of the “innate” immune
system, such as those seen
attached to the cancer cells
prior to their death.
A Big Surprise: Cancer
Resistance in These Mice is
Dependent on Age
 When
enough SR/CR mice were bred
to create a larger colony of
these mice, other types of
experiments were done. The
original cancer resistance
occurred in mice that were
six weeks old --just
post-adolescent for a
mouse). However, it was now
possible to wait longer
before injecting cancer
cells to see how well older
mice responded. When testing
for cancer resistance was
delayed until 5 months of
age, the mice that had
inherited the resistance
gene began to grow tumors,
just as normal mice do.
However, when the tumors
reached a detectable size at
2-3 weeks, many of these
mice showed a sudden
decrease in tumor size in a
day or two, followed by
disappearance of the tumor
completely. In some cases,
this “spontaneous
regression” of cancer
(Figure 3) was quite
dramatic -- a very large
tumor mass disappeared
overnight. What appeared to
be happening in these older
mice was that the cancer
could grow until the
anti-cancer mechanism
finally “kicked” in,
ultimately killing all of
the cancer cells. When
enough SR/CR mice were bred
to create a larger colony of
these mice, other types of
experiments were done. The
original cancer resistance
occurred in mice that were
six weeks old --just
post-adolescent for a
mouse). However, it was now
possible to wait longer
before injecting cancer
cells to see how well older
mice responded. When testing
for cancer resistance was
delayed until 5 months of
age, the mice that had
inherited the resistance
gene began to grow tumors,
just as normal mice do.
However, when the tumors
reached a detectable size at
2-3 weeks, many of these
mice showed a sudden
decrease in tumor size in a
day or two, followed by
disappearance of the tumor
completely. In some cases,
this “spontaneous
regression” of cancer
(Figure 3) was quite
dramatic -- a very large
tumor mass disappeared
overnight. What appeared to
be happening in these older
mice was that the cancer
could grow until the
anti-cancer mechanism
finally “kicked” in,
ultimately killing all of
the cancer cells.
If these spontaneously
regressing mice were then
re-injected with new cancer
cells, they appeared now to
be completely resistant.
Thus, they had been primed
by the prior rejection of
tumor cells. In a sense,
they had been vaccinated
against cancer. These primed
mice can be repeatedly
injected with cancer cells
and remain resistant
throughout their normal
lifespan. If, however, the
mice reached the age of 1
year before being exposed
for the first time to cancer
cells most of these mice
were not cancer resistant,
even though they clearly had
the gene mutation (since
their offspring were
resistant when tested at an
early age). This surprising
result is discussed further
in the next section, under
the topic: why does cancer
incidence rise with
increasing age?
When Cancer Cells Die in the
SR/CR Mice, How Does It
Happen?
The failure of cancer to
grow in the completely
resistant young SR/CR mice
could be due to some
property of the mice that
prevented growth of tumor
cells, or it could be due to
active killing of cancer
cells even though they could
initially grow. The
spontaneous regression seen
in older SR/CR mice, in
fact, strongly pointed to a
killing mechanism, rather
than just preventing growth.
When the cancer cells of the
resistant mice were
recovered, the cancer cells
showed rupture of the cell
surface membrane, a process
referred to as cytolysis.
Other experiments showed
that this rupture probably
involves toxic proteins that
are manufactured and
secreted by cells of the
immune system. Two of these
toxic proteins (perforin and
granzyme B) were found in
the fluid around the cancer
cells.
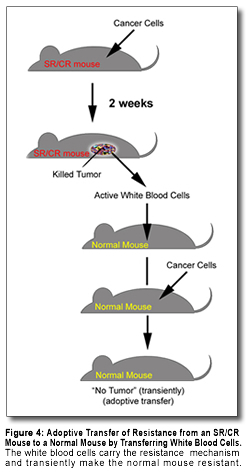 A
further important question
in understanding how these
mice killed cancer cells was
whether the killing required
live cells from the
resistant mouse, or could be
mediated by some floating
molecule independent of
intact immune cells. One way
of studying this issue is to
isolate cells involved in
the killing mechanism from
the resistant mouse and
transfer them either into a
normal mouse (Figure 4), or
into a test tube with living
cancer cells. In both of
these tests, the cells from
the resistant mice killed
cancer cells, but the
soluble materials did not.
This strongly suggests that
cells of the mouse directly
attack and kill cancer
cells. It also shows that
resistant immune cells can
be transferred to a normal
mouse and, at least
transiently, make that
normal mouse
cancer-resistant. This
experiment, called 'adoptive
transfer,' could be the
model for a similar approach
to treat cancer in people if
such resistant immune cells
could be generated in large
numbers. A
further important question
in understanding how these
mice killed cancer cells was
whether the killing required
live cells from the
resistant mouse, or could be
mediated by some floating
molecule independent of
intact immune cells. One way
of studying this issue is to
isolate cells involved in
the killing mechanism from
the resistant mouse and
transfer them either into a
normal mouse (Figure 4), or
into a test tube with living
cancer cells. In both of
these tests, the cells from
the resistant mice killed
cancer cells, but the
soluble materials did not.
This strongly suggests that
cells of the mouse directly
attack and kill cancer
cells. It also shows that
resistant immune cells can
be transferred to a normal
mouse and, at least
transiently, make that
normal mouse
cancer-resistant. This
experiment, called 'adoptive
transfer,' could be the
model for a similar approach
to treat cancer in people if
such resistant immune cells
could be generated in large
numbers.
Another test is to remove
the immune cells from the
mouse and see whether cancer
could now grow. When this
“immunodepletion” experiment
was done, and more of the
immune cells were removed,
the mice gradually lost
their resistance to cancer.
However, when the depletion
treatments were stopped, the
mouse regained its immune
system and the tumor
regressed. This is direct
evidence that the killing of
cancer cells in these mice
is due to cells of the
immune system.
SR/CR Mice are Healthy and
Live a Normal Lifespan
The type of mouse in which
this resistance mechanism
was first studied, BALB/c
mice, have a normal lifespan
of around 2 years. An
important question in the
study of the cancer
resistance mechanism was
whether the resistant mice
were healthy. So far,
studies of these mice have
not shown any shortening of
their lifespans. In fact,
the original mouse with this
trait which had been
injected with large numbers
of cancer cells many times
during its life lived to be
26 months old and had many
offspring.
Another important question
is whether the ability to
resist cancer is accompanied
by some other disease
problem. For example,
special genetically altered
mice have been developed
with highly active immune
systems that can reject
tumors, but they usually
also show evidence of
rejecting normal tissue
cells, a process referred to
as “autoimmunity.”
Autoimmune mechanisms are
the basis for several
serious human diseases, such
as lupus erythematosus and
rheumatoid arthritis.
However, the SR/CR mice have
shown no signs of these
autoimmune complications.
The cancer resistant
mechanism in these mice is
surprisingly selective,
apparently only affecting
cancer cells. This selective
property is of great
interest to scientists
studying these events, since
it suggests that such
selectivity can actually
exist and be the basis of
future anti-cancer
therapies.
The SR/CR Mouse Mutation as
a “Handle” to Study Cancer
Resistance
Since the resistance seen in
the SR/CR mice is an
inherited trait, and appears
to involve a mutation in a
single gene, then the
identification of the
mutation and the gene which
contains it can provide
important clues as to how it
might work. Since all of the
genes of the mouse and the
human have been identified
from the various genome
projects in the last few
years, it will be possible
to examine the exact
mutation in the mouse and to
correlate it with possible
changes in similar genes in
people. Mice and people have
similar immune systems. It
is likely that if we can
understand the mechanism
that is used by this
mutation in mice, we will be
able to apply this knowledge
to identify and manipulate
similar mechanisms in
patients. The key to this
study is the exact nature of
the mutation present in
these mice. Multiple studies
are underway to identify
this mutation and its
consequences. While there
are no guarantees that we
can use this knowledge to
treat human cancer, we can
speculate on the future
secrets that this
information could reveal, as
described in the next
section.
|

